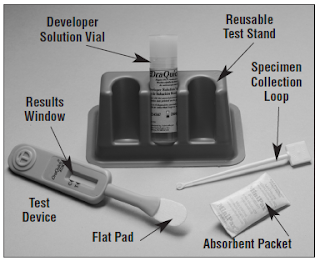
In the early 1980’s when AIDS was still unexplained and HIV
was spreading unchecked so was HCV. Both agents are blood borne viruses that
struck intravenous drug users and blood transfusion recipients. In 1989 Hepatitis C virus was identified and
now estimates are about 130-200 million people are affected worldwide. About
35,000 people worldwide die every year from HCV related liver disease. 30% of
people infected with HIV are also infected with HCV as well. Many of the
antiretrovirals used to treat HIV build up in poisoning the organ the HCV drugs
are trying to Save. “Their differences are greater than their similarities.”
Says Miriam Alter an epidemiologist at the university of texas medical branch
in galveston. 80% of people affected with HIV know they have it yet only 30% of
people with HCV know they have it. Worldwide number drops to 5% “it doesn’t
matter how good your treatments are if the majority of your affected population
is not diagnosed” Gregory Dore Head of viral hepatitis clinical research
program at university of new south Wales in Sydney. One of the more recent
developments in testing for HCV has been the development of rapid test for
anti-HCV. The OraQuick HCV Rapid Antibody Test was approved by the FDA in February
2011. As we know presence of the antibody does not mean that the person is
infected with the virus but only shows exposure to the virus. Further testing
will need to be done to confirm current infection. But the development of this
assay allows for faster screening of larger numbers of people and can be used
to rule out infection. For additional information on this assay refer to http://www.orasure.com/docs/pdfs/products/hcv/us/OraQuick_HCV_Package_Insert.pdf
Monday, July 30, 2012
Smallpox in Birmingham!
On August 11, 1978 Janet Parker, a medical photographer at University
of Birmingham Medical School, in the United Kingdom, fell ill with headache and
pains in her muscles. She begins to develop a rash that the doctors assume is
just a benign rash. Not until 14 days later does she finally get confirmation
on the true cause of her illness. She has contracted Variola major, the worst
form of the virus causing smallpox. On September 11, 1978, one month after
first becoming ill, she dies becoming the last person to die from smallpox. Her
mother became infected but did not die from smallpox. Two Biomedical Scientist,
the equivalent to a medical technologist in England, were also quarantined
until October 10, 1978. It was determined that she contracted the virus from
faulty ventilation from the laboratory on a floor below that was conducting
research on the virus. I think this makes a very good case for the importance
of laboratory safety. Ironically enough thought she had been vaccinated for
smallpox in 1966. But the problem with the vaccine is initial vaccination only
provides a high level of protection for 3 to 5 years. In 1796 Edward Jenner
discovered that a person could be immunized from smallpox by being exposed to
cowpox. Cowpox along with the Variola virus is a member of the Poxviridae
family. Current vaccines use the Vaccinia virus which is also a member of
Poxviridae. Talking of smallpox reminded me of one of my favorite episodes of
House, a medical television show that aired on the Fox network. The episode was
titled “A Pox on Our House” and if you ever get a chance to watch it I
recommend it(http://www.youtube.com/watch?v=-sq5iXIc1fw).
So now to what makes it difficult for medical professional like you and I to
watch medical programs. In the episode they are fearful that the father of the
suspected infected girl has also been exposed to the virus so he is given the
vaccine by the CDC. Dr. House who has decided that the girl doesn’t have
smallpox becomes confused when the father starts to develop a rash very similar
to smallpox. The brilliant Dr. House decides that the father must have contracted
smallpox from the vaccine. So there lies what we should all have a problem
with. The vaccine is made up of a live virus but it is the Vaccinia virus and
not the Variola virus. Not to say that the vaccine doesn’t have problems but
you cannot get smallpox from the vaccine.
Monday, July 23, 2012
VITROS 5600 Part 2
Last post I discussed some of the properties of the VITROS 5600 integrated system. So lets say you have reviewed my previous post decided you have room for this 2300 pound 9 foot wide instrument in your laboratory. I know you want one for your bedroom as well so how much will it cost you? The list price for the VITROS 5600 integrated system is $410,000 but don’t write that check just yet. According to one report by the ECRI institute, they also list other factors that will affect cost such as service contract, which was quoted at $23,546.25 per analyzer per year and a shipping cost of $1,400.00 per analyzer. Reagent pricing will vary depending on many factors but they quoted a price for the ECi A-HCV as $4.18 per test. ECRI institute recommends that prices be negotiated in order to decrease cost of the analyzer, reagents, service, and shipping. The VITROS 5600 employs 5 different measurement principles: Colorimetric/Rate, Potentiometric (direct ISEs), Immuno-rate, Turbidimetric, and Enhanced Chemiluminescence. The VITROS 5600 offers over 120 available assays. The principle behind the assay for HCV is Enhanced Chemiluminescence. The assay is FDA approved for in vitro qualitative detection of immunoglobulin G antibody to hepatitis C virus (anti-HCV) in human serum and plasma. No reagent preparation is needed for VITROS 5600. The VITROS 5600 integrated system offers advantages including the variety of assays included in the menu and automation. However this system has disadvantages such as initial high cost and a large footprint for the size of the instrument.
Don't get TB!
Having done my annual Tuberculosis skin test the day before we talked about it in class got my attention a little. I know we talked about it some in immunology so some of this may be a review. The Mantoux tuberculin skin test is used to determine if a person is infected with Mycobacterium tuberculosis. First step in the test is 0.1 ml of tuberculin purified protein derivative is injected in the inner surface of the forearm with the bevel of the needle facing up. The time frame in which the test is read is critical to correctly identifying a positive test. The test must be read after 48 hours but before 72 hours. So you may remember from class that the area is measured in millimeters to determine a positive test. What is measured is the raised hardened area or area of swelling and not any redness. I initially thought the report I was given was reported out incorrectly because they reported it as 0.0mm when there was clearly a small area of redness at the site of injection but the area if redness is not measured it is the raised area of which I had none. What is considered positive? According to the CDC 5mm or more in: HIV-infected persons, a recent contact of a person with TB disease, persons with fibrotic changes on chest radiograph consistent with prior TB, patients with organ transplants, or persons who are immunosuppressed for other reasons. If you have 10mm or more and Recent immigrants (< 5 years) from high-prevalence countries, injection drug users, residents and employees of high-risk congregate settings, mycobacteriology laboratory personnel, persons with clinical conditions that place them at high risk, children < 4 years of age, or infants, children, and adolescents exposed to adults in high-risk categories are also considered positive. Additionally anyone with test result greater than 15mm, even with no known risk factors. Some potential false positives include: Infection with nontuberculosis mycobacteria, previous BCG vaccination, incorrect method of TST administration, incorrect interpretation of reaction, or incorrect bottle of antigen used. False negatives can result from the following: Cutaneous anergy (anergy is the inability to react to skin tests because of a weakened immune system), recent TB infection, very old TB infection, very young age, recent live-virus vaccination, overwhelming TB disease, some viral illnesses, incorrect method of TST administration, or incorrect interpretation of reaction. So be careful and don’t get TB once your out working please!
Monday, July 16, 2012
VITROS 5600 Part 1
One method for the detection of HCV is the VITROS 5600 integrated system by Ortho Clinical Diagnostics (Raritan, NJ). This summary will examine the instruments application, methodology and performance characteristics in general for the instrument and as it relates to HCV testing. The VITROS 5600 integrated system is produced by Ortho Clinical Diagnostics. Ortho Clinical Diagnostics is a part of the Johnson and Johnson Family of companies. The company has roots that date back to 1937 with the establishment of the Ortho Product division by Johnson and Johnson. In 1989 Ortho Clinical introduced the first assay for the detection of antibodies to hepatits C virus. In 2001 they became the first company to receive U.S. Food and Drug Administration approval for automated random access hepatitis test, performed on the VITROS ECi Immunodiagnostic system.
The Vitros 5600 is an integrated system that integrates immunoassay testing and clinical chemistry testing all in one platform. This high-capacity system is the first of its kind. The instrument was cleared by the FDA in October 2008 and there is currently greater than 900 units in clinical use worldwide.
The first thing to be reviewed will be application characteristics of the VITROS 5600. The instrument is 2.79m /109.7inches wide, 0.85m/33.5 inches in depth, 1.64m/64.5 inches in height, and weighs 1062kg/2340 pounds. The power requirements for voltage are two dedicated 20 amp power lines or one dedicated 30 amp power line with UPS, nominal 200-240V AC and the requirements for frequency is 47-63Hz. Environmental requirements include operating temperature 15°-30°C, ambient relative humidity 15%-75% RH, and altitude of up to 8,000 feet. The instrument does not require a water source or a drain. It is designed with a self-contained onboard waste management system in order to eliminate requirements for off board plumbing. The instrument generates 60 decibels of noise at idle and 65 while operational. The operator interface is a color coded graphical interface that uses an ergonomic flat low-glare 17inch touchscreen LCD. There is a numeric keypad that is also included on the monitor. In order to provide maximum flexibility the included keyboard is detachable. The time it takes for a single result will vary depending on the principal of the assay being performed. The range is as short as about 2.5 minutes for potentiometric assays and as long as about 16 to 73 minutes for microwell assays. In the case of the anti-HCV assay the estimated time for results is less than 60 minutes. The typical delay from ordering a stat test to aspiration of the sample is about 10 seconds. Sample types include serum, plasma, urine, CSF, whole blood, and amniotic fluid (not available in US). The type of sample will be dependent on the assay being performed. The anti-HCV assay is approved for serum and plasma samples. Sample volumes range from 2-180 µL. Samples can be continuously be loaded and unloaded as instrument is in operation and can hold 80 samples in universal sample trays and 10 samples in dedicated STAT lane. Stay tuned for more to come on the VITROS 5600.
I get all the best Porin Channels!
Porins are proteins that are located in the outer membrane of gram negative bacteria. The porin functions as a channel from outside of the cell to the periplasm. Periplasm is the region between the outer and inner membranes of the bacteria. Porin channels vary in the size of the pore in the cell. Bacteria typically have a variety of porins that are important for the transport of nutrients needed by the cell. Passage of antibiotics into gram negative cells occurs through the porin channels. Many bacteria have developed resistance to antibiotics by altering the porin channel so that the antibiotic can no longer pass into the cell. For example the porin OprD is utilized for the transport of imipenem into the bacteria. In some strains of Pseudomonas aeruginosa they have altered the structure of the OprD porin channel and as a result become resistant the antibiotic imipenem. Some other strains that have developed resistance include: Enterobacter aerogenes and Klebsiella spp. against imipenem, Vancomycin intermediate-resistant S. aureus or VISA strains with thickened cell wall trapping vancomycin, Many Gram-negative bacteria against aminoglycosides, and Many Gram-negative bacteria against quinolones.
Monday, July 9, 2012
Gold for the win!
The American University in Cairo
are working on a test to detect HCV RNA in a single reaction without amplifying
the RNA first. Using gold nanoparticles . Gold particles have an unusual
optical property known as surface plasmon resonance. When particles are
distributed evenly in liquid they reflect light in a way that makes them appear
red. When they clump together they appear blue. Patient serum and Short pieces
of DNA designed to match the HCV RNA is added to solution the Gold
nanoparticles added. If HCV RNA is present the DNA pieces will bind with it.
And the gold particles will clump together appearing blue. If the HCV RNA is
not present then the DNA pieces will bind to the Gold particles preventing them
from clumping resulting in the solution turning red. The test cost about 1/7
the cost of the current HCV RNA test and only takes about 30 minutes.
With the increasing number of
diagnostic technologies the CDC has begun discussion with the world health
organization about collaborating to create standards to ensure that results of
different test can be compared. In developed countries like the US the overall prevalence mass
screening is not cost effective. The current CDC guidelines were developed in
the late 1990’s recommend testing those known to have been at risk of exposure
to the virus. But a lot of the people who contracted the virus several decades
ago don’t realize they are at risk and do not come forward to be tested
In the united states 1 in 33 baby
boomers might be infected according to a model designed by Lisa McGarry of i3
Innovus part of health care information technology company Ingenix.
With this in mind the CDC may
release recommendations in the guidelines for age
based screening.
I hear you!
As most of you know I have a 2 year old daughter. About a year ago she began to have chronic ear infections with fluid buildup in her ear. Ear infections are more common in children because the Eustachian tube is shorter and horizontal. This makes it easier for bacteria to enter and the smaller diameter hinders the movement of fluids. The Eustachian tube is a tube that links the nasopharynx to the middle ear. Additionally inflammation of the tube due to infection can cause swelling and trap fluid in the ear allowing bacteria to grow in the fluid. Some of the signs of infection are fever, irritability, and ear pain as a result of the buildup of pressure. Hearing loss may also result from the buildup of pressure but most children do not have long term affects to their hearing. Antibiotics can be given to treat ear infections but they may resolve on there own. In our case most of the time a round of antibiotics would clear up the ear infection within a week. In a few cases we had to change antibiotics in order to resolve the problem. This became an ongoing process with about 1 to 2 ear infections per month. Our pediatrician recommended a more invasive procedure of having myringotomy tubes put in. myringotomy tubes more commonly known as ear tubes are small tubes made of plastic, metal, or Teflon. The tube is inserted through a incision made into the eardrum while the child is under general anesthesia. The insertion of these myringotomy tubes allow the trapped fluid to flow out of the middle ear. Since we had the procedure done this past January we have only had one ear infection.
Sunday, July 1, 2012
Just being me!
With an estimated 5.2 million people
infected with Hepatitis C Virus (HCV) you probably know someone that is infected. A lot
of famous people have come out and stated that they are infected with HCV. One
of the more famous people that claimed to be infected with HCV is Keith Richards.
Keith Richards is most famously known for being the founding member of The
Rolling Stones. Keith Richards in relation to Hepatitis C Virus is noted for
his statement that he was cured of HCV by "just being me." So you
probably don't believe him but what if I told you that it very well could be
true. In fact 14 to 40% of those exposed to HCV spontaneously clear the virus.
Some of the major factors associated with spontaneous clearance include a
single exposure, younger age at infection, female sex, and certain HLA genes.
Additionally African Americans and Asian Americans appear to be less likely
than Hispanic or white Americans to clear the virus spontaneously. Of those who
do not clear the virus and progress into chronic Hepatitis C Virus there are
some treatment options. The treatment itself is one of the main problems
associated with Hepatitis C virus. The current treatment for chronic Hepatitis
C virus is pegylated interferon along with ribivarin. The results of this
expensive treatment only cure patients approximately 50% of the time and have toxic
side effects associated with it. Multiple factors have been found to be
associated with response to this treatment including viral and host factors.
The viral factor that has been found to be the best predictor of response to
treatment is virus genotype, with genotype 2 and 3 reaching 70% to 80%
sustained response. Several commercial assays for HCV genotyping are available
for use in clinical laboratories.
Subscribe to:
Comments (Atom)